Hyperbarric Oxygen Therapy in Rehabilitation of Cerebral Palsys
- Corresponding Author:
- Norihan Abdelaziz Elgazar
Physical Medicine
Rheumatology and Rehabilitation Medicine
Assiut University, Egypt
E-mail: wafaagad2015@gmail.com
Abstract
The study included 60 cerebral palsy patients attending the outpatient clinic of Physical Medicine, Rheumatology and Rehabilitation Tanta University Hospital. They were assessed and divided into two groups: Group 1: (30 patients) referred to Hyperbaric Medical Institution in Tanta university hospital to receive HBOT 40 sessions, in addition to their conventional rehabilitation program for six months. Group 2: (30 patients) received their conventional rehabilitation program only in the form of sessions of physical therapy three times per week for the treatment period (six months). mp3download.link Best YouTube to MP3 converter. Download MP3 from YouTube for Free. one would honestly have expected more on the track mp3-go.net Download Mp3 songs for free Given the legendary pedigree of the man behind the sound downloadmp3-gratis.biz Download mp3 songs online at Mp3 Converter, watch high quality online music videos download-mp3gratis.me watch and download free songs of the highest quality. Listen to songs online here comfortably without any annoying advertisements. metrolagu.com Easy to use and free MP3 downloader. YouTube To MP3 download in seconds using the best YouTube to MP3 converter. YouTube Mp3 Get the latest song by simply typing the latest artist or song title in the Search menu. Mp3 file format with 128 - 320 Kbps bitrate converted from YouTube videos. read at this blog All those artist performances are still available on YouTube today find more here
Keywords
HBOT; Rehabilation; Oxygen therapy
Introduction
CP is a static encephalopathy which means brain injury that is non-progressive disorder of posture and movement of variable etiologies that maybe associated with epilepsy, speech problems, vision compromise and cognitive dysfunction [1,2].
CP is caused by damage to the motor control centers of the developing brain and can occur during pregnancy, childbirth or after birth up to three years. The incidence of CP increases with premature or very low-weight babies regardless of the quality of care [3-6].
Many impairments of sensation, perception, cognition, communication, behavioral problems, epilepsy, difficulties with sleeping, drooling and feeding accompany motor disorders in CP and require medical management. Therefore, health care for children with CP requires the skills of variety of professions who must work efficiently and collaboratively with the family as team.
The goal of rehabilitation is to gain independence in activities of daily living school or work and social life. Child rehabilitation aimed to improve mobility, prevent deformity and educate the parents about the child’s problem. It also involves helping the child to learn the skills he will need in daily life in school and while playing with friends. Lastly rehabilitation means decreasing the complications which arise as a result of the child’s neuro-muscular impairments.
Rehabilitation of CP children include traditional physiotherapy as electrotherapy, hydrotherapy, heat therapy, massage, therapeutic exercises, stretching exercises and functional therapy as hippo therapy and vojta technique which prescribed to prevent contractures and to strengthen the weak muscles to enable functional use of the upper extremity and to establish a better walking pattern. Also the current rehabilitation programs of CP start using hyperbaric oxygen in addition to other rehabilitation programs because some clinical trials of using Hyperbaric Oxygen Therapy (HBOT) in CP have reported significant improvement in study groups [7,8].
HBOT involves inhaling 100% oxygen at greater than one Atmosphere Absolute (ATA) in a pressurized chamber. HBOT has been used successfully in humans at varying pressures to treat a range of conditions. Many clinical applications of HBOT are including treatment of decompression sickness, arterial gas embolism, carbon monoxide poisoning, amyotrophic lateral sclerosis, and complex regional pain syndrome. However, HBOT has also been used at lower pressures (1.5 ATA or less) with clinical success in conditions including ischemic brain injury and CP [8].
Therapeutic effects of HBOT in CP are due to an increase in dissolved oxygen in plasma and tissue that help tissue to regenerate which used to treat CP under the theory that improving oxygen availability to damaged brain cells can reactivate some of them to function normally but Its use to treat CP is controversial [8-10].
Material and Methods
The study included 60 cerebral palsy patients attending the outpatient clinic of Physical Medicine, Rheumatology and Rehabilitation Tanta University Hospital. They were assessed and divided into two groups:
â Group 1
(30 patients) referred to Hyperbaric Medical Institution in Tanta university hospital to receive HBOT sessions, in addition to their conventional rehabilitation program for six months. (Hyperbaric oxygen therapy was performed in mono chamber hyperbaric chamber from SECHRIST Company Para med made in USA) [11].
• Patients was compressed and decompressed at 2-3 psi/minute (pounds per square inch) with air, the rate depending on patient comfort and tolerance
• Depth of pressurization with 1.5 Absolute Atmosphere ATA
• Total oxygen breathing time is 60 minutes with 5 minutes’ air break after 30 minutes’ oxygen breathing
• Treatment was once/day, 5 days/week for 40 sessions
â Group 2
(30 patients) received their conventional rehabilitation program only in the form of sessions of physical therapy three times per week for the treatment period (six months).
Physical therapy and rehabilitation program that was done in both groups using different types of physical modalities 3 times per each week in addition to exercises at home.
Physical modalities in the form of
• Excersise (active, assisted active or passive)
• Massage to limbs affected
• Stretching of tendoachilis
• Heat therapy in the form of infrared rays, hot packs [12]
â Inclusion criteria
• CP diagnosed according to Scientific neurological examination to early detect delay in developmental mile stones
• CP children with age group up to 5 years
• CP children with negative family history of degenerative brain insults
• CP children with absent any deformities of any other musculoskeletal diseases
â Exclusion criteria
• Patients not fit for hyperbaric oxygen therapy as
• Patients with active epilepsy and recurrent seizers
• Patients with tension pneumothorax
• Patients with uncontrolled heart failure
• Eustachian tube dysfunction
• Patient with rupture ear drum
• Patient on mechanical ventilation
• Children of CP aged more than 5 years
• CP children with positive family history of degenerative brain insults
• Children with other deformities of any other musculoskeletal diseases that limits movements
• Children with behavioral problems
• Children who were treated with botulinum toxin or orthopedic surgery within the past 6 month or dorsal rhizotomy within the past 2 years
• Deaf and blind children
â All Patients will be assessed by
The following data was determined for each patient at time of start of the study. Complete history taking from parents or child caregiver by asking them about
• Demographic data: (name-age-sex)
• Past history: as epilepsy, asthma, etc
• Prenatal history: To detect any abnormalities during pregnancy period that predispose to CP
• Natal history: Type of delivery to detect any delay of labor causing insufficient oxygen supply to brain of baby
• Post-natal history: Accident or infection of case before 3 years that predispose to CP [13,14]
• Family history to exclude any case of positive family history of congenital brain diseases
• Consanguinity of parents of patient
• Clinical examination: At start of study, 3 months later and at the end of the study
Results
Our study showed insignificant difference between 2 studied groups at 3 and 6 months in relation to gross and fine movement and reduction of spasticity.
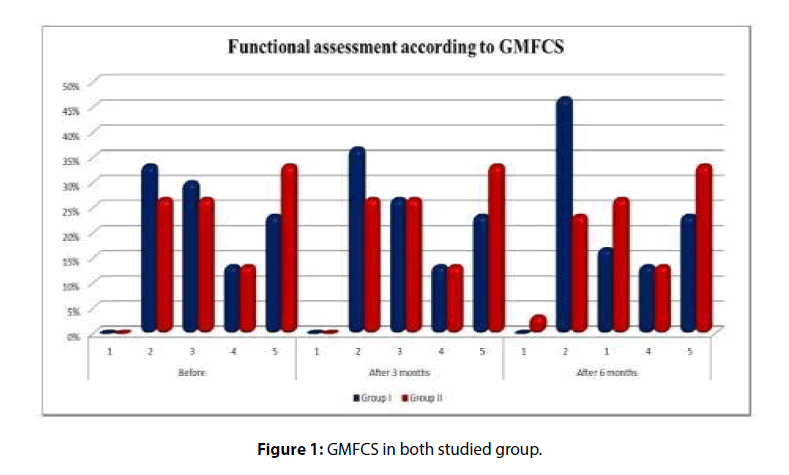
All children in both groups were assessed in GMFCS according to their ages. There was insignificant difference between both groups before treatment, 3 months and 6 months of study according to GMFCS. There was improvement in both groups at 3 and 6 months follow up compared to before treatment but this improvement was statistically insignificant with more improvement in group 1 (Table 1 and Figure 1).
Table 1: Functional assessment of both studied groups (according to GMFCS) at the start of the study, 3 and 6 months follow up.
| GMFCS | Groups | Chi-square test | |||||
| Group I | Group II | X2 | p-value | ||||
| n | % | n | % | ||||
| Before | 1 | 0 | 0 | 0 | 0 | 0.909 | 0.908 |
| 2 | 10 | 33.3 | 8 | 26.7 | |||
| 3 | 9 | 30 | 8 | 26.7 | |||
| 4 | 4 | 13.33 | 4 | 13.3 | |||
| 5 | 7 | 23.33 | 10 | 33.3 | |||
| After 3 Months | 1 | 0 | 0 | 0 | 0 | 1.094 | 0.81 |
| 2 | 11 | 36.66 | 8 | 26.7 | |||
| 3 | 8 | 26.66 | 8 | 26.7 | |||
| 4 | 4 | 13.33 | 4 | 13.3 | |||
| 5 | 7 | 23.33 | 10 | 33.3 | |||
| After 6 Months | 1 | 0 | 0 | 1 | 3.3 | 3.811 | 0.395 |
| 2 | 14 | 46.66 | 7 | 23.3 | |||
| 3 | 5 | 16.66 | 8 | 26.7 | |||
| 4 | 4 | 13.33 | 4 | 13.3 | |||
| 5 | 7 | 23.33 | 10 | 33.3 | |||
| B-3M | FrX2 | 1.000 | - | ||||
| p-value | 0.317 | - | |||||
| 3-6M | FrX2 | 2.000 | 1.000 | ||||
| p-value | 0. 157 | 0.317 | |||||
| B-6M | FrX2 | 3.000 | 1.000 | ||||
| p-value | 0.083 | 0.317 | |||||
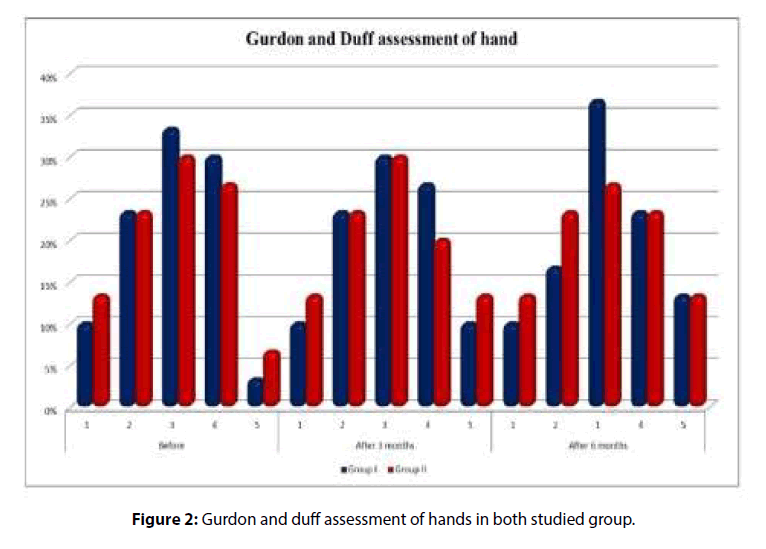
6 months according to Gurdon and Duff at 3 and 6 months follow up compared to before treatment but this improvement was statistically insignificant with more improvement in group 1 (Table 2 and Figure 2).
Table 2: Functional assessment of upper limbs of both studied groups at the start of the study, 3 and 6 months follow up (Gurdon and Duff assessment of hand).
Gurdon and Duff assessment of hand |
Groups | Chi-square test | |||||
| Group I | Group II | X2 | p-value | ||||
| n | % | n | % | ||||
| Before | 1 | 3 | 10 | 4 | 13.3 | 0.588 | 0.964 |
| 2 | 7 | 23.33 | 7 | 23.3 | |||
| 3 | 10 | 33.33 | 9 | 30 | |||
| 4 | 9 | 30 | 8 | 26.7 | |||
| 5 | 1 | 3.33 | 2 | 6.7 | |||
| After 3 Months | 1 | 3 | 10 | 4 | 13.3 | 0.571 | 0.966 |
| 2 | 7 | 23.33 | 7 | 23.3 | |||
| 3 | 9 | 30 | 9 | 30 | |||
| 4 | 8 | 26.66 | 6 | 20 | |||
| 5 | 3 | 10 | 4 | 13.3 | |||
| After 6 Months | 1 | 3 | 10 | 4 | 13.3 | 0.950 | 0.917 |
| 2 | 5 | 16.66 | 7 | 23.3 | |||
| 3 | 11 | 36.66 | 8 | 26.7 | |||
| 4 | 7 | 23.33 | 7 | 23.3 | |||
| 5 | 4 | 13.33 | 4 | 13.3 | |||
| B-3M | FrX2 | 2.00 | 2.00 | ||||
| p-value | 0.157 | 0.157 | |||||
| 3-6M | FrX2 | 1.00 | 1.00 | ||||
| p-value | 0.317 | 0.317 | |||||
| B-6M | FrX2 | 3.00 | 2.00 | ||||
| p-value | 0.083 | 0.157 | |||||
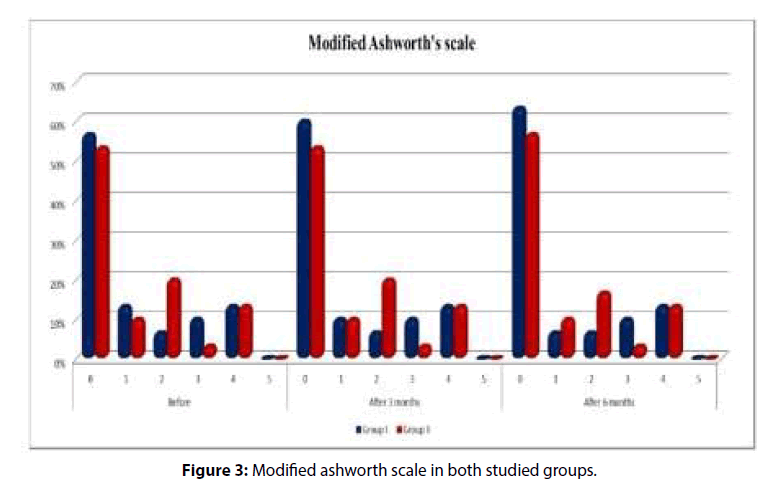
There was insignificant difference between both groups before treatment,3 months and 6 months according to MAS. There was improvement in both groups at 3 and 6 months follow up compared to before treatment but this improvement was statistically insignificant with more improvement in group 1 (Table 3 and Figure 3).
Table 3: Modified Ashworth’s scale of both groups at the start of the study, 3 and 6 months follow up in both studied groups.
Modified Ashworth |
Groups | Chi-square test | |||||
| Group I | Group II | X2 | p-value | ||||
| n | % | n | % | ||||
| Before | 0 | 17 | 56.66 | 16 | 53.3 | 3.124 | 0.547 |
| 1 | 4 | 13.33 | 3 | 10 | |||
| 2 | 2 | 6.66 | 6 | 20 | |||
| 3 | 3 | 10 | 1 | 3.3 | |||
| 4 | 4 | 13.33 | 4 | 13.3 | |||
| 5 | 0 | 0 | 0 | 0 | |||
| After 3 Months | 0 | 18 | 60 | 16 | 53.3 | 3.096 | 0.568 |
| 1 | 3 | 10 | 3 | 10 | |||
| 2 | 2 | 6.66 | 6 | 20 | |||
| 3 | 3 | 10 | 1 | 3.3 | |||
| 4 | 4 | 13.33 | 4 | 13.3 | |||
| 5 | 0 | 0 | 0 | 0 | |||
| After 6 Months | 0 | 19 | 63.33 | 17 | 56.7 | 2.594 | 0.665 |
| 1 | 2 | 6,66 | 3 | 10 | |||
| 2 | 2 | 6.66 | 5 | 16.7 | |||
| 3 | 3 | 10 | 1 | 3.3 | |||
| 4 | 4 | 13.33 | 4 | 13.3 | |||
| 5 | 0 | 0 | 0 | 0 | |||
| B-3M | FrX2 | 1.00 | - | ||||
| p-value | 0.317 | - | |||||
| 3-6M | FrX2 | 1.00 | 1.000 | ||||
| p-value | 0.317 | 0.317 | |||||
| B-6M | FrX2 | 2.00 | 1.00 | ||||
| p-value | 0.157 | 0.317 | |||||
Discusion
In relation to gross motor function follow up of both groups at 3 and 6 months, there was improvement occur at 3 and 6 months compared to the start of treatment in both groups with insignificant difference between the 2 groups [15-17] but slightly better in group 1 suggesting that the role of HBOT in improving motor function of CP after 40 sessions is of no significant importance as the result of study of Waalkes et al. [18] that was designed to assess the effect of HBOT as an adjuvant therapy in CP showing non-significant improvements in motor function and spasticity and observed that mild improvements were placebo effects and therefore, HBOT therapy had no neuro therapeutic effects on TBI and CP also as the result of Lacey et al. [19] that is against using HBOT in rehabilitation of CP but this is against the result of Sethi et al. [20] which concluded that HBOT associated with occupational therapy and physiotherapy is effective in improving the motor skills of children with CP that can be explained that The combined action of hypoxia and hyperbaric pressure leads to improvement in tissue oxygenation and affects both oxygensensitive and pressure-sensitive genes. HBO2 therapy can initiate vascular repair and improve cerebral vascular flow, induce regeneration of axonal white matter, stimulate axonal growth, promote blood-brain barrier integrity and reduce inflammatory reactions as well as brain edema [21].
According to fine motor assessment that measured via Gurdon and Duff assessment that was done to assess hand function to allow doctors to follow the improvement of hand function of CP, there was insignificant difference between 2 studied groups at start of the study.
In relation to hand function assessment at 3 and 6 months’ follow-up, no significant difference between improvement in both studied groups as the result of the study of Waalkes et al. [18] that suggest that by observing children at sessions of HBOT there was no effect of it in improving fine movement by hands.
Also our result can be explained by short time receiving sessions of HBOT as there are debate about minimum and maximum duration to receive HBOT in CP as weeks to months are necessary for brain tissue regeneration and angiogenesis, but the upper time limit from which no further improvements are expected remains unknown.
Conclusion
Physical therapy is still the first line of rehabilitation in CP that must be started as early as possible to improve gross and fine motor dysfunction. HBOT of minor role in improving motor delay and disabilities of CP.
Conflict of Interest
There is no actual or potential conflict of interest in relation to this article.
References
- Jindal P, Rosenbaum P, Direzze B, et al. Treatment and rehabilitation of children with cerebral palsy in India. Dev Med Child Neuro 61, 1050-1060 (2019).
- Adam T, Juan C, Mark P, et al. Symptoms burden in individuals with cerebral palsy. J Rehabil Res Dev 47, 863-867 (2010).
- Hiltz D, Thurman J, Gwinn-I-lardy K, et al. How common are the n common neurologic disorders? Neurology 68, 326-337 (2007).
- Ferrari A, Cioni G. The spastic forms of cerebral palsy: A guide to the assessment of adaptive functions. ASJC Italy 88, 3-15 (2010).
- Tommiska V, Heinonen K, Lehtonen L, et al. No improvement in outcome of extremely low birth weight infant population between 1996-1997 and 1999-2000. J Paediatrics 19, 29-36 (2007).
- Svraka E, Loga S, Brown I. Family quality of life: Adult school children with intellectual disabilities in Bosina and Herzegovina. J Intellect Disabil Res 55, 365-388 (2011).
- Gabb G, Robin ED. Hyperbaric oxygen: A therapy in search of diseases. J Chest 92, 1074-1082 (1987).
- Waisman D, Shupak A, Melamed Y, et al. Hyperbaric oxygen therapy in the pediatric patient. The experience of the israel naval medical institute. Pediatrics 102, e53 (1998).
- Hadanny A, Abbott S, Efrati S, et al. Effect of hyperbaric oxygen therapy on chronic neurocognitive deficits of posttraumatic brain injury patients: Retrospective analysis. BMJ 8, 23387 (2018).
- Raelina S, Theresa C, Woods M, et al. Hyperbaric oxygen therapy: Indications, contraindications, and use at a tertiary care center. Aorn J 107, 443-450 (2018).
- Butler C, Campbell S. Evidence of the effects of intrathecal baclofen for spastic and dystonic cerebral palsy AACPDM treatment outcomes committee review panel. Dev Med Child Neurol 42, 34-45 (2000).
- McDonagh M, Morgan D, Carson S, et al. Systematic review of hyperbaric oxygen therapy for cerebral palsy: The state of the evidence. Dev Med Child Neurol 49, 942-947 (2007).
- Farmer J, Sabbagh A. Selective dorsal rhizotomies in the treatment of spasticity related to cerebral palsy. Child Nerv Sys 23, 991-1002 (2007).
- Gretl l, Rocky f, Ernest S, et al. Hyperbaric oxygen therapy: Exploring the clinical evidence advances in skin and wound care. Adv Skin Wound Care 107, 442-453 (2017).
- Hanft B, Pilkington K. Therapy in natural environments: The means or end goal for early intervention? Infants Young Child 12, 1-13 (2000).
- Azhar M, Zareen A, Saleem Z, et al. Evaluation of role of hyperbaric oxygen therapy in children with cerebral palsy: Our experience at armed forces hospital, King Abdul Aziz naval base, KSA. Paediatrics J 11, 1407-1411 (2017).
- Wang Y, Zhu Y, Qiu B, et al. Study on the effectiveness of hyperbaric oxygen nursing and treatment for children with cerebral palsy. Acta Medica Mediterranea J 35, 657:660 (2019).
- Waalkes P, Fitzpatrick DT, Stankus S, et al. Adjunctive HBO treatment of children with cerebral anoxic injury. US Army Med Dep J 13-21 (2002).
- Lacey D, Stolfi A, Pilati L. Effects of hyperbaric oxygen on motor function in children with cerebral palsy. Ann Neurol 72, 695-703 (2012).
- Sethi A, Mukherjee A. To see the efficacy of hyperbaric oxygen therapy in gross motor abilities of cerebral palsy children of 2-5 years, given initially as an adjunct to occupational therapy. Indian Occup J 1, 7-12 (2003).
- Carole S, Serge L, Pierre M, et al. Hyperbaric oxygenation therapy in treatment of cerebral palsy: A review and comparison to currently accepted therapies. J of Amer Physi and Surg 12, 109-113 (2007).