Comparative Study of Pathogenic Factors of Mycoplasma agalactiae Isolates
- Corresponding Author:
- Islam M Wassif
Animal Health Department
Animal, and Poultry Production Division
Desert Research Center, Egypt
E-mail: islam_wassif@hotmail.com
Abstract
Abstract
Protein profiles of selected M. agalactiae isolates of goats were carried out, it was clear that there were variations between selected strains. There was a similarity only in two monomorphic bands which were about 57 kDa and about 29 kDa. In addition to 6 polymorphic bands which were 113 kDa, 87 kDa, 75 kDa, 49kDa, 43 kDa and 36 kDa. There were 7 unique protein bands for each strain, 121 kDa (PG2), 108 kDa (strain 2), 91 kDa (PG2), 70 kDa (strain 2), 65 kDa (PG2), 63 kDa (strain 3) and 35 kDa (strain 2). On the other hand, results of the Western blot test revealed that reference strain 1 and strain 4 had immunogenic bands at Molecular weight of 57 kDa and 49 kDa. The strain 2 had immunogenic bands at M. wt of 57 kDa and 46 kDa. The strain 3 which recovered from normal caprine milk had not the immunogenic band.
Keywords
Protein profile; Immunogenic band; Antigenic characterization
Introduction
Studies on protein and antigenic characterization must be conducted in order to choose the strains for vaccine preparation against M. agalactiae [1]. Contini et al. described highly immunogenic surface lipoproteins of Mycoplasma agalactiae in the range 45-55 kDa [2]. Bergonier et al. reported that variable expression has been demonstrated by monoclonal antibodies in a low kDa range surface proteins of M. agalactiae strains [3]. Solsona et al. studied the genomic, protein profile and antigenic profile of Mycoplasma agalactiae isolates from different geographic areas in France using pulsed-field gel electrophoresis, sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting [4]. They observed that M. agalactiae appeared to be genetically stable but antigenically variable in addition to that they could not identify any particular difference between virulent and “supposedly” a virulent strains. They claimed that antigenic variability was to some extent related to the geographic origin of the strains and was of less importance than the variability of the phylogenetically similar species M. bovis. They concluded that this more limited variability could result either from very special socio-economic conditions (quite isolated enzootic areas without any outside exchange) or from really different genetic determinisms. Tola et al. investigated the genomic variability of 81 Mycoplasma agalactiae isolates from different Italian outbreaks using pulsed field gel electrophoresis, to compare its results with the protein and antigenic profiles obtained on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and on immunoblotting [5]. They reported that isolates were all similar without intraspecific differences by genetic analysis and this homogeneity was confirmed by immunoblotting. 80 and 50 kDa antigens were present in all strains analyzed. Tola et al. demonstrated that highly immunogenic surface lipoproteins of Mycoplasma agalactiae in the range of 45-55 kDa. Therefore, the protein and antigenic profiles of the M. agalactiae strains (reference strain and three representative local field isolates of M. agalactiae, two from diseased goats, strains 2, 4 and strain 3 which recovered from apparently healthy goat) were analyzed using SDS-PAGE and Western blot test [6].
Materials and Methods
• Strains M. agalactiae strains (reference strain and three representative local field isolates of M. agalactiae, two from diseased goats, strains 2, 4 and strain 3 which recovered from apparently healthy goat) were analyzed using SDS-PAGE and Western blot test
• Determination of total protein concentration of M. agalactiae antigen [7]
• Protein analysis of M. agalactiae isolates by SDS-PAGE [8]
• Western blot of M. agalactiae isolates [9]
Results and Discussion
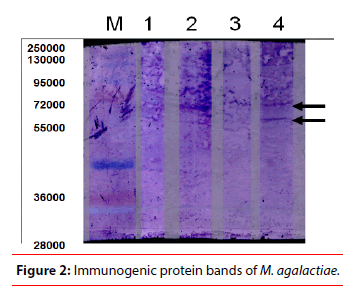
Concerning the SDS-PAGE, the reference strain PG2 (strain 1) in addition to three selected local isolates of M. agalactiae (strain 2 isolated from diseased goats, strain 3 isolated from apparently healthy animal and strain 4 isolated from diseased goat) were subjected to SDS-PAGE for investigation of protein profile as shown Figure 1 and Table 1 for protein analysis. It was clear that there was variation between protein profiles of the selected strains. This observation was in good agreement with the study of De-la Fe et al. who reported that comparing electrophoretic wholecell protein profiles of M. agalactiae isolates showed antigenic variability to an extent not observed by other workers [10]. These results could be due to the sophisticated mechanism of mycoplasmas to vary the antigenic repertoire of their cell surface, overcoming differences in the expression of surface proteins, and which is used to circumvent the host immune system [11- 14]. On the other hand, a 55 kDa protein band described as one of the most constant proteins of M. agalactiae [15,16]. This presumably corresponded to the common monomorphic band reported in the present investigation and having an apparent molecular weight of 57 kDa. Another monomorphic protein band was seen at 29 kDa. The same band was observed by Solsona et al. who stated that however protein profile of M. agalactiae clearly appeared fairly homogeneous, but this protein homogeneity seemed to be in contradiction with the great antigenic heterogeneity revealed by immunoblotting. In addition to 6 polymorphic protein bands which were observed at 113 kDa, 87 k Da, 75 k Da, 49 kDa, 43 kDa and 36 kDa, as shown in Table 1, which simulate what was observed by Firouzi et al. who studied the protein profiles of three local strains of M. agalactiae in Iran. In the present study, there were 7 unique protein bands for each strain as shown in Table 2, 121 kDa (PG2), 108 kDa (strain 2), 91 kDa (PG2), 70 kDa (strain 2), 65 kDa (PG2), 63 kDa (strain 3) and 35 kDa (strain 2). Data collected in the present investigation revealed a high degree of protein variability among the selected local strains of M. agalactiae. Glew et al. characterized a multigene family (vspma genes) undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins that are subject to phase and size variation in M. agalactiae [17]. Consequently, the observed polymorphic pattern in the protein profile of the selected strains was related to the genetic polymorphism in vspma genes. Thus, the finding of stable, specific and strongly immunogenic antigens using Western blot test is necessary in order to improve detection, identification and vaccine elaboration against this agent [18]. In the immunoblotting study, the immunogenic proteins were found in a molecular weight range between 46 and 57 kDa which was more concordant with results obtained by Tola et al. 1997, who detected the major immunogenic proteins between 40 and 90 kDa, but in contrast to findings of Solsona et al. 1996 who reported a molecular weight range between 25 and 36 kDa [4,5]. These authors also observed some correlation between the antigenic profiles of French M. agalactiae strains and their geographical origin. Such a relationship could not be derived from the data of the present study. Western blotting with pooled sera collected from natural infected goats with M. agalactiae detected expression of three immunodominant protein bands (46, 49 and 57 kDa) as shown in Figure 2 and Table 2 which agree with Levisohn et al., who detected three immunogenic bands at molecular weight of 41, 47 and 50 kDa of their local M. agalactiae isolates. In the present work, the reference strain and strain 4 had immunogenic bands at M. wt of 57 kDa and 49 kDa [19]. The strain 2 had immunogenic bands at M. wt of 57 kDa and 46 kDa as shown in Figure 2 and Table 2. On the other hand, strain 3 which recovered from normal caprine milk, had not any immunogenic band. Recently it was considered that the immunogenic bands in a molecular weight range between 45 and 55 kDa were expressed as P48 which was described as an invariable, constantly expressed, immunodominant, surface lipoprotein and belongs to the basic membrane protein family (virulent factor of M. agalactiae) [20]. They added that the P48 does not fall in the size range of variable surface antigens and mechanisms of variation, such as inversion, insertion, repetition of polymeric sequences, frameshift mutations, have not been found at the genetic level. By analogy with the homologous P48 of M. fermentans, this lipoprotein plays a fundamental immunomodulating role during early infection [21,22]. It had been proven to be associated with high levels of specificity and sensitivity [23]. In the present investigation, the absence of P48 from strain 3 which recovered from normal caprine milk, supports the finding that P48 is a characteristic for virulent strains of M. agalactiae. P48 represents an interesting antigen for further studies on virulence of M. agalactiae. From this point, it was clear that the two local isolates from diseased animals (strains 2 and 4) have virulent factors in their antigenic structure. Thus, it was considered as good candidates for vaccine preparation against M. agalactiae infection. M, Marker, lane 1 (strain 1) reference strain of M. agalactiae (PG2), lane 2 (strain 2) M. agalactiae local isolate recovered from diseased animal), lane 3 (strain 3) M. agalactiae local isolate recovered from app. healthy animal) and lane 4 (strain 4) M. agalactiae local isolate recovered from diseased animal.
| Lane | Marker | Lane 1 | Lane 2 | Lane3 | Lane 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Row | M. wt. | Rel Mobility | M. wt. | Rel.Mobility | M. wt. | Rel Mobility | M. wt. | Rel Mobility | M. wt. | Rel Mobility |
| 1 | 250000 | 0.1937 | ||||||||
| 2 | 121754.3 | 0.2617 | ||||||||
| 3 | 130000 | 0.2395 | 113468.5 | 0.2664 | 113468.5 | 0.2664 | ||||
| 4 | 108363.2 | 0.2814 | ||||||||
| 5 | 95000 | 0.3028 | 91463.25 | 0.3146 | ||||||
| 6 | 87875.09 | 0.3233 | 87875.09 | 0.3233 | ||||||
| 7 | 72000 | 0.366 | 75140.34 | 0.3609 | 75140.34 | 0.3609 | ||||
| 8 | 70718.24 | 0.3771 | ||||||||
| 9 | 65456.27 | 0.3992 | ||||||||
| 10 | 63597.22 | 0.4079 | ||||||||
| 11 | 55000 | 0.4601 | 57017.86 | 0.4433 | 57017.86 | 0.4433 | 57017.86 | 0.4433 | 57017.86 | 0.4433 |
| 12 | 49942.72 | 0.4917 | 49942.72 | 0.4917 | 49942.72 | 0.4917 | ||||
| 13 | 46800.86 | 0.5178 | ||||||||
| 14 | 43612.35 | 0.5483 | 43612.35 | 0.5483 | ||||||
| 15 | 36000 | 0.6522 | 36974.1 | 0.6284 | 36974.1 | 0.6284 | ||||
| 16 | 35072.41 | 0.6569 | ||||||||
| 17 | 28000 | 0.7913 | 29193.92 | 0.7688 | 29193.92 | 0.7688 | 29193.92 | 0.7688 | 29193.92 | 0.7688 |
Table 1: Analysis of protein profile of local and reference strains of M. agalactiae by using SDS-PAGE.
| Lane 1 | Lane 2 | Lane 3 | Lane 4 |
|---|---|---|---|
| PG2 | Strain 2 | Strain 3 | Strain 4 |
| 57017.86 | 57017.86 | - | 57017.86 |
| 49942.72 | 46800.86 | - | 49942.72 |
Table 2: The molecular weight (kDa) of immunogenic bands revealed by Western blot test.
M, Marker, lane 1 (strain 1) reference strain of M. agalactiae (PG2), lane 2 (strain 2) M. agalactiae local isolate recovered from diseased animal), lane 3 (strain 3) M. agalactiae local isolate recovered from app. healthy animal) and lane 4 (strain 4) M. agalactiae local isolate recovered from diseased animal.
Conclusion
Results of the Western blot test revealed that reference strain 1 and strain 4 had immunogenic bands at Molecular weight of 57 kDa and 49 kDa. The strain 2 had immunogenic bands at M. wt of 57 kDa and 46 kDa. The strain 3 which recovered from normal caprine milk had not the immunogenic band. From this point, it was clear that the two local isolates from diseased animals (strains 2 and 4) have virulent factors in their antigenic structure. Thus, it was considered as good candidates for vaccine preparation against M. agalactiae infection.
References
- De-la Fe, C Assuncao, P Rosales, et al. Protein and antigen variability among Mycoplasma mycoides subsp. mycoides (LC) and Mycoplasma agalactiae field strains by SDS-PAGE and immunoblotting. Vet J 171, 532-538 (2006).
- Contini A, Pittau M, Cuccuru C, et al. A study of an experimental infection of sheep with Mycoplasma agalactiae studies of antibodies response. New Microbiol 22, 27-30 (1999).
- Bergonier D, De-Simone F, Russo P, et al. Variable expression and geographic distribution of Mycoplasma agalactiae surface epitopes demonstrated with monoclonal antibodies. FEMS Microbiol Lett 143, 159-165 (1996).
- Solsona M, Lambert M, Poumarat F. Genomic, protein homogeneity and antigenic variability of Mycoplasma agalactiae. Veter Micro 50, 45-58 (1996).
- Tola S, Idini G, Manunta D, et al. Comparison of Mycoplasma agalactiae isolates by pulsed field gel electrophoresis, SDS-PAGE and immunoblotting. FEMS Microbiol Lett 143, 259-265 (1996).
- Tola S, Manunta D, Cocco M, et al. Characterization of membrane surface proteins of Mycoplasma agalactiae during natural infection. FEMS Microbiol Lett 154, 355-362 (1997).
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with Folin phenol reagent. J Biol Chem 193, 265-275 (1951).
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685 (1970).
- Towbin, H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications Proc. Natl Acad Sci USA 76, 4350-4354 (1979).
- De-la Fe C, Assuncao P, Saavedra P, et al. Field trial of two dual vaccines against Mycoplasma agalactiae and Mycoplasma mycoides subsp. mycoides (large colony type) in goats. Vaccine 25, 2340-2345 (2007).
- Rottem S, Naot Y. Subversion and exploitation of host cells by mycoplasmas. Trends in Microbiol 6, 436-440 (1998).
- Yogev D, Rosengarten R, Watson-Mckown R, et al. Molecular basis of Mycoplasma surface antigenic variation. A novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 50 regulatory sequences. The EMBO Journal 10, 4069-4079 (1991).
- Simecka JW, Davis JK, Davidson MK, et al. Mycoplasma diseases of animals. Molecular biology and pathogenesis. Amer Society Microbiol 391-416 (1992).
- Pfutzner H, Sachse K. Mycoplasma bovisas an agent of mastitis pneumonia, arthritis and genital disorders in cattle. Revue Scientifique et technique (International Office of Epizootics). 1477-1494 (1996).
- Flitman-Tene R, Levisohn S, Lysnyansky E, et al. A chromosomal region of Mycoplasma agalactiae containing vsp-related genes undergoes in vivo rearrangement in naturally infected animals. FEMS Microbiol Lett 191, 205-212 (2000).
- Santona A, Carta F, Fraghi P, et al. Mapping antigenic sites of an immunodominant surface lipoprotein of Mycoplasma agalactiae, AvgC, with the use of synthetic peptides. Infect Immun 70, 171-176 (2002).
- Firouzi R, Hosseini MH, Homa Orangi, et al. Immunochemical study of protein profiles of Taleghan, Fars, and Lorestan strains of Mycoplasma agalactiae. Comp Clin Path 20, 39-42 (2009).
- Glew MD, Papazisi L, Poumarat F, et al. Characterization of a multigene family undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins in Mycoplasma agalactiae. Infect Immun 68, 4539-4548 (2000).
- Levisohn S, Flitman-Tene R, Rapoport E. Genetic variation in Mycoplasma agalactiae strains isolated in Israel. Mycoplasmas of ruminants. Pathogenicity, diagnostics, epidemiology and molecular genetics. COST 826, Belgium 3, 9-11 (1999).
- Rosati S, Pozzi S, Robino P, et al. P48 major surface antigen of Mycoplasma agalactiae is homologous to a malp product of Mycoplasma fermentans and belongs to a selected family of bacterial lipoproteins. Infect Immun 67 , 6213-6216 (1999).
- Matsumoto M, Nishiguchi M, Kikkawa S, et al. Structural and functional properties of complement-activating protein M161Ag, a Mycoplasma fermentans gene product that induces cytokine production by human monocytes. J Biol Chem 273, 12407-12414 (1998).
- Calcutt MJ, Kim MF, Karpas AB, et al. Differential posttranslational processing confers intraspecies variation of a major surface lipoprotein and a macrophage-activating lipopeptide of Mycoplasma fermentans. Infect Immun 67, 760-771 (1999).
- Rosati S, Robino P, Fadda M, et al. Expression and antigenic characterization of recombinant Mycoplasma agalactiae P48 major surface protein. Vet Microbiol 71, 201-210 (2000).