Anterior Aesthetic Zone Reconstruction with Autogenous Bone Graft from the Mandibular Ramus using a Modiï¬Âed Shell Technique for Dental Implant Placementâ Case Report
- Corresponding Author:
- Sghaier Jihed, Monastir 5000, Tunisia, Faculty of Dental Medicine, Department of Medicine and Oral Surgery, University Of Monastir,
Phone : +21627585527,
E-mail: Sghaier.jihed25@gmail.com
Received: 26-Jan-2024 ; Manuscript No IJOCS-24-125626; Editor Assigned: 29-Jan-2024, PreQC No. IJOCS-24-125626 (PQ); Reviewed: 10-Feb-2024, QC No. IJOCS-24-125626 (Q); Revised: 19-Feb-2024, Manuscript No. IJOCS-24-125626 (R); Published: 20-Mar2024, DOI: 10.37532/1753- 0431.2024.18(1).242
Abstract
Bone atrophy after tooth loss may leave insufficient bone for implant placement. Several reconstructive procedures for the maxilla have been proposed to increase alveolar bone dimensions in both the vertical and horizontal directions. Alveolar bone grafting from an intraoral donor site offers a successful treatment option to regain the original bone volume.
The present article describes the case of a 30-year-old male who reported to the Department of Oral Medicine and Oral Surgery at the University Dental Clinic of Monastir- Tunisia, for replacement of maxillary incisors 21 and 22, lost due to a traffic accident complicated by significant bone loss.
The patient underwent autogenous bone grafting using a modified Shell Technique, and autogenous bone was harvested from the mandibular ramus. The shells were trimmed to a thickness of 1 mm and placed to recontour the ideal shape of the alveolar ridge. The shells were then fixed with micro titanium screws, and the gap between the shells and the alveolar ridge was filled with a mixed xenograft and autogenous bone chips.
Postoperative follow-up was favorable, with considerable vertical and horizontal bone gain. This article aims to shed light on the importance of the Modified Shell Technique for vertical and horizontal bone provision and how it remains the "gold standard" for reconstructing bone loss, especially in the maxillary anterior region
Keywords
Alveolar ridge augmentation, Bone regeneration, Alveolar bone loss, Alveolar bone grafting, Mandibular nerve, Cone-beam computed tomography
Introduction
Horizontal and vertical alveolar bone loss hinders dental implant placement [1]. Advancements in bone reconstruction techniques have enhanced both aesthetic and functional outcomes. However, restoring the oral function of atrophic alveolar crests remains a challenge in oral implantology [2]. Autogenous block graft reconstruction of alveolar defects before implant surgery is still regarded as the gold standard [1].
Modified Shell Technique has received broad interest since its inception. Khoury F [3]. described the shell technique for threedimensional hard tissue grafting. Thin cortical bone shells, harvested with a special cutting wheel from the retromolar region, were placed to reshape the alveolar crest and to protect the particular bone (set in the cavity between the shells), from resorption. Harvesting the bone shells and extra oral trimming with a cutting wheel is very technique-sensitive. Additional harvesting of bone chips is also necessary. Therefore, the authors modified and simplified this technique [3].
The technique described in this article used one donor site to harvest the bone graft. The corticocancellous bone graft was thinned out using a bone mill. At this moment, the thickness of the cortical bone shell could be easily controlled. In addition, the shape of the shell was slightly convex, corresponding to the shape of the alveolar crest because of the bone mill’s circular rasp. Bone chips were also harvested simultaneously and mixed with xenograft bone. The following case report details the surgical procedure stepby-step.
Case Presentation
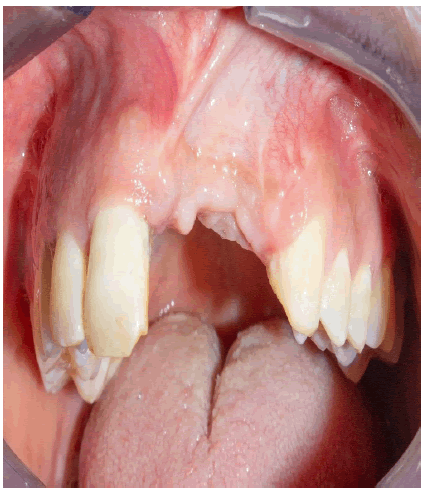
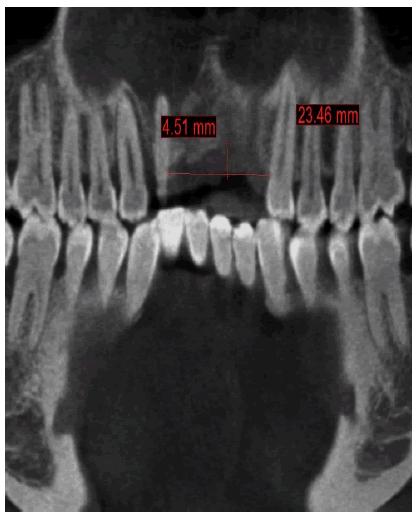
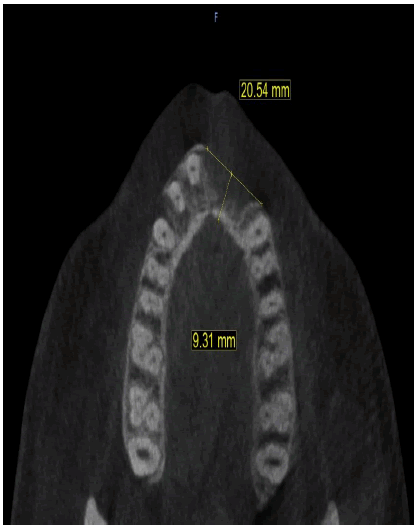
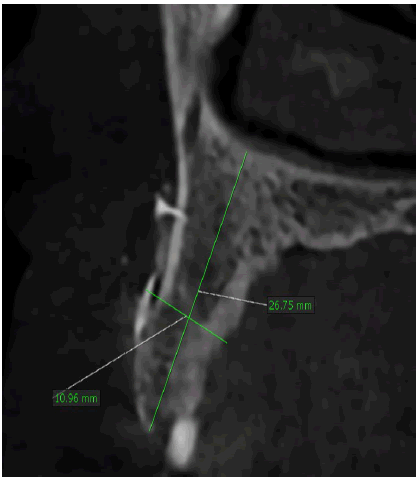
A healthy 30-year-old male reported to the Department of Oral Medicine and Oral Surgery at the University Dental Clinic of Monastir-Tunisia asking to replace his missing left central and lateral incisors due to a traffic accident, probably with implants. A Cone Beam Computed Tomography (CBCT) is advised to know bone height, width, and density at the proposed implant sites. CBCT revealed only 7.27 mm bone width and only 11.5mm bone height at the implant sites (Figure 1a-c).
This vertical and horizontal bone loss necessitated bone augmentation to allow adequate implant placement for optimal aesthetic and functional results.
The requirements of the Declaration of Helsinki were observed, and the patient gave informed consent for all surgical procedures.
The surgical procedure was performed under local anesthesia.
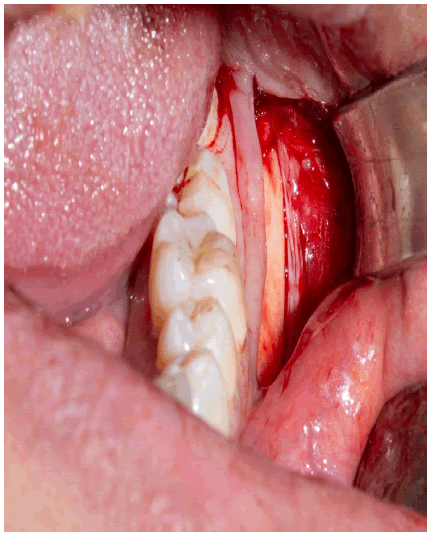
A mid-crestal incision with the bilateral oblique releasing incision is given in the maxillary anterior region on the future implant site, and the mucoperiosteal flap’s full thickness is elevated. The bone defect was measured using a periodontal probe to determine the defect and the graft’s sizes.
Then, a full-thickness incision was made para marginally to the right mandibular molars. A full-thickness flap was elevated to permit visualization of the external oblique ridge. The ramus donor site was lateral to the molar and extended up to the ascending ramus.
A superior osteotomy was created approximately 4mm-5mm medial to the external oblique ridge with the help of a piezo surgery kit using an OT7 insert. It begins distal to the first molar and continues posteriorly to the ascending ramus. The length of this osteotomy depends on the graft size. The vertical osteotomies start at each end of the superior bone cut and continue inferiorly, approximately 11mm -12mm, with the help of an OT8L insert. Finally, an OT8R insert was used to create a second horizontal osteotomy connecting each vertical osteotomys inferior aspect (Figure 2a).
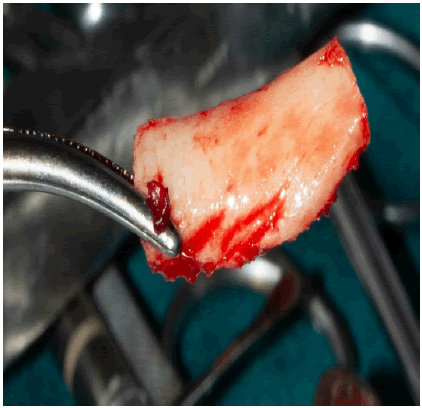
The graft was then harvested using malleted bone spreader along the superior osteotomy (Figure 2b). The harvested bone block had a thickness of approximately 3mm.
The recipient site was perforated with 0.8mm round blur to access tubercular bone blood vessels to the graft and accelerate revascularization.
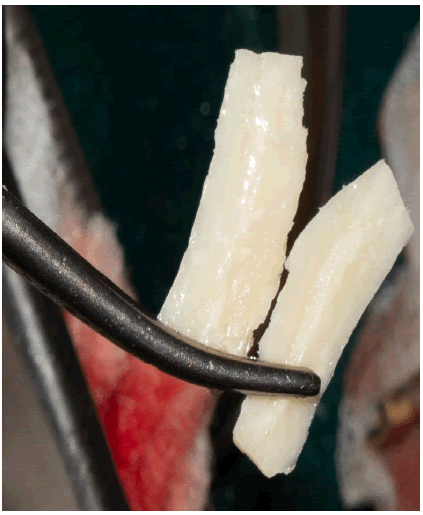
The bone block was then split lengthwise using a thin diamond disk under copious irrigation with saline solution (Figure 3).
The two plates obtained were secondly adapted with a cutting wheel, adjusted with a round blur, to the recipient site at vestibular and palatal positions.
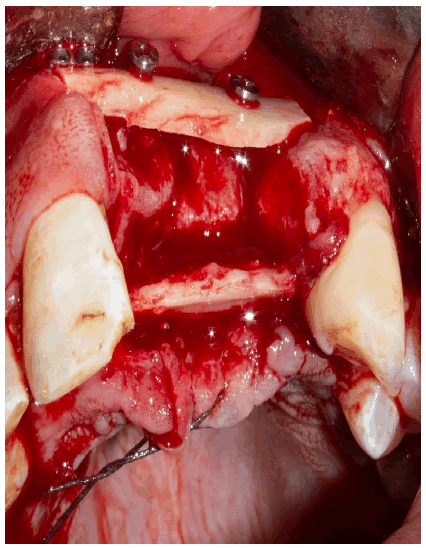
The first plate was attached to the vestibular bone table with two titanium microscrews, 2 mm in diameter x13 mm; then the second plate is attached to the palatal table with two osteosynthesis screws 2 mm in diameter x10 mm (Figure 4).
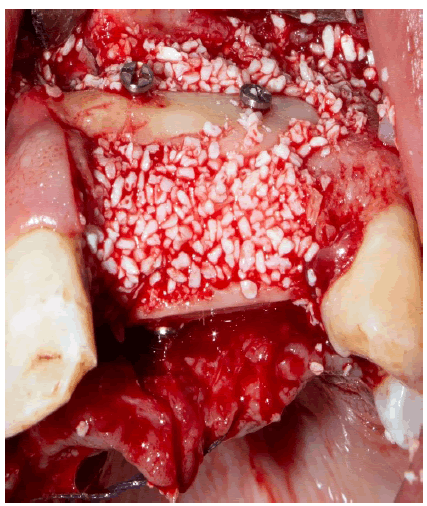
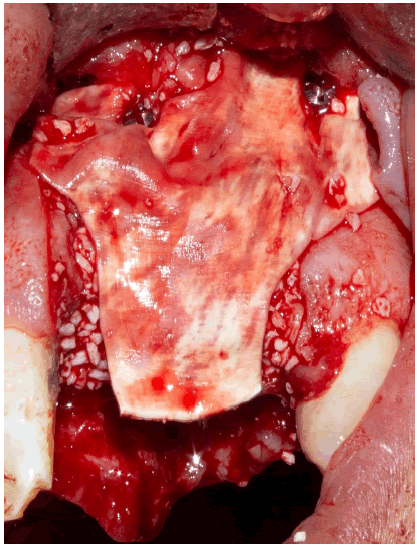
The bony envelope formed in this way was then filled with bovine allogeneic bone chips mixed with autogenous bone chips and patient blood (Figure 5).
Finally, the bone graft was covered with a resorbable collagen membrane secured by two pins at the vestibular level (Figure 6).
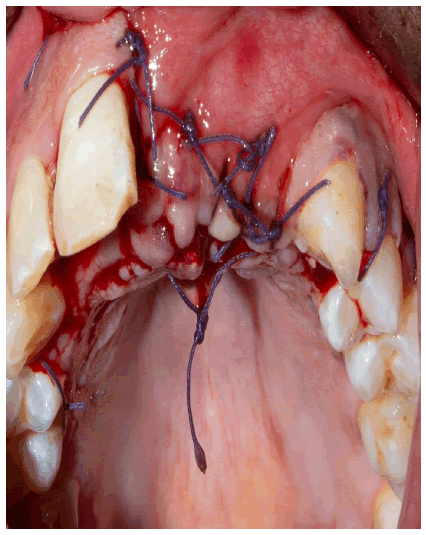
A periosteal incision allowed flap release and tension-free wound closure with a coronally advanced flap. To secure the wound margins, deep horizontal mattress sutures were used for wound fixation, and interrupted sutures were applied for wound adaptation (Figure 7).
Sutures were removed 14 days after surgery, and the wound was wholly closed without any signs of inflammation (Figure 8).
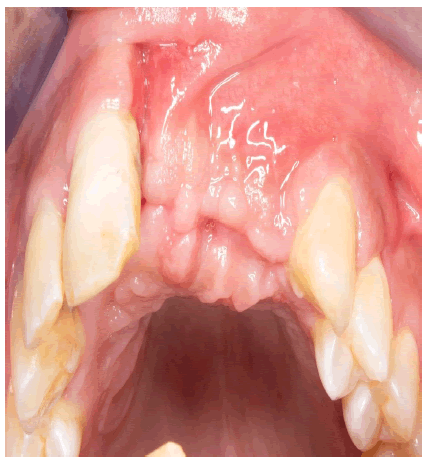
To document the surgical result, a CBCT was obtained just 4 months after surgery. (Figure 9).
The patient was provided with a Removable Dental Prosthesis (RDP). Particular attention was paid so that no contact or pressure from the prosthesis was transferred to the gingiva or the graft.
Reentry was programmed 6 months after the surgical procedure.
Discussion
Alveolar ridge deficiency following tooth loss compromises oral implant rehabilitation [4]. When the alveolar process dimensions are insufficient, horizontal and vertical alveolar ridge augmentation is commonly required prior to implant insertion [5,6].
The anterior portion of the oral cavity is the most difficult from an aesthetic standpoint. In many cases, the horizontal bone volume must be augmented due to partial or full bone loss [7].
Several grafting methods have been developed to generate enough bone volume for implant insertion [8].
Bone grafts harvested from an intraoral site provide better results when compared to grafts harvested from the calvaria or iliac crest [9].
Grafts from the ramus of the mandible are a convenient source of autogenous bone for alveolar reconstruction [7].
The benefits of using autologous bone harvested for alveolar reconstruction must be carefully evaluated, as most studies have focused primarily on the reconstructive procedure at the recipient site or on harvesting complications, with only a small number of studies reporting the final results of the augmentation procedure [10, 11]. Bone harvested from the mandible appears to have biological benefits because of its embryologic origin [12,13]. This is also attributed to the low rate of complications [14]. The most major issues with autogenous complete block transplants documented in the literature are 21%- 25% resorption rates and limited availability [15, 16].
The external oblique ridge is favorable to obtain a large mandibular bone block graft, Radiography should be used to provide additional information on the donor site and its relationship to vital neighboring anatomical structures [17].
Authors have observed that block grafts with a thicker cortex and a higher density, such as those taken from the calvarium or mandible, will typically reveal higher stability compared to grafts exhibiting a thin cortex, such as the tuberosity or the iliac crest [18- 20].
Conversely, the cancellous portion of the block graft has an important function in stimulating osteogenic cells to differentiate into osteoblasts to form new bone. In addition, the cancellous block graft portion re-vascularizes more quickly than cortical bone grafts [21]. Therefore, combining both characteristics in cortico-cancellous block grafts promotes early vascularization with maximum graft maintenance at the same time [21].
Long-term studies assessing horizontal and vertical alveolar ridge augmentation with autogenous bone block have demonstrated implant survival of 95.7% and 98.1%, after 10 years [22, 23].
The survival of supra structures after 3D ridge augmentation with Bio-Oss alone or combined with Particulate Autogenous Bone Graft (PABG) has not been reported in comparative or non-comparative studies. Neither has the survival of supra structures after 3D ridge augmentation with autogenous bone block [5].
Consequently, further randomized controlled studies assessing the survival of supra structures after 3D ridge augmentation with different surgical techniques and graft materials are needed before any conclusions concerning the final implant treatment outcome can be provided [5].
The 3-year Implant survival was 96% After 3D ridge augmentation with 50% Bio-Oss Mixed with 50% PABG. The 3-year Implant survival with autogenous bone block was previously reported as 90%. Thus, 3D ridge augmentation with a mixture of 50% Bio-Oss and 50% PABG Appears to demonstrate a similar implant survival rate with an autogenous bone block in the short term [24].
Von Arx and Buser recommended covering the bone block graft with inorganic bovine bone and a collagen membrane to enhance guided bone regeneration. After 6 months, resorption of 7.5 mm and an overall increase of 4.6 mm in graft width were seen in their research [10].
However, as collagen membranes have a relatively short duration of barrier function, the block grafts must be protected from surface resorption by other means. In a recent clinical comparative study, Maiorana et al. have shown the beneficial effect of block graft coverage using ABBM particles [25]. They reported resorption of only 9.3% for sites treated with ABBM particles, whereas sites without such coverage demonstrated bone resorption of 18.3% [25].
Cordaro et al. reported mean horizontal and vertical augmentations of 5.0 mm ± 0.23 mm and 2.2 mm ± 0.6mm, respectively, after mandibular block grafting with no membrane for protection [26].
Autologous bone block grafts have a greater flexibility, as they can be used in all clinical situations (also in cases of complex). On the other hand, they show a more significant potential for post-operative morbidity (risk of graft infection and neurologic sequelae related to bone harvesting in intra-oral site). Harvesting grafts from the mandibular region may cause neurological complications due to the risk of damage to the inferior alveolar nerve. A sensory deficit in the lower lip and mental areas of 8.3% in mandibular ramus harvesting was reported, compared with 16% for the chin as the donor site [27].
The healing mechanisms and incorporation of autogenous block bone grafts are universal regardless of the donor site [28].
The osteocytes within these grafts die off because of their encasement in a mineral matrix and disrupted their delicate canalicular blood supply. New bone is formed by osteogenesis as a result of surviving endosteal osteoblasts and marrow stem cells, which are few in block grafts and by osteoinduction from the release of BMP and IGF-1 and -2 as the mineral matrix is resorbed and by osteoconduction through the framework of the graft itself. Block bone grafts form new bone, mostly by osteoinduction and osteoconduction from the adjacent bone margins and much less through direct osteogenesis from surviving osteocompetent cells [28].
It has been suggested that a thin biotype may compromise the collateral blood supply to the underlying osseous structures, whereas a thick biotype may enhance it. Flap management and surgical trauma may also influence the degree of primary and collateral blood supply to the underlying bone block graft, and ischemia may result from a lack of adequate new angiogenesis [29].
In one retrospective review of esthetic outcome 18.9 months after immediate implant placement, sites with a thin tissue biotype had a higher frequency of recession ± 1 mm compared to thick sites [30].
Many studies on intraoral alveolar bone harvesting have focused mainly on the reconstructive procedure at the recipient site or the complications related to harvesting. Only a minimal number of studies have reported the results of the harvesting procedure [31]. Harvesting mandibular symphysis bone grafts by piezoelectric surgery was found to significantly decrease the incidence of sensory disturbance of both the skin and the oral mucosa and to reduce pulp damage in the adjacent teeth [32].
Recent studies have reported that both ramus and symphysis harvesting procedures are well accepted by patients but the ramus procedure is generally preferred [33].
Autogenous bone grafting has several advantages over other augmentation techniques, including short healing times, favorable bone quality, lower material costs, no risk of disease transmission or antigenicity, and predictability in repairing larger defects or greater atrophy. Denser cortical bone grafts exhibit minimal resorption on incorporation, making them ideal for site development [34].
The timing of implant placement after reconstruction of alveolar ridges by autogenous bone block graft technique remains a controversial topic [18].
Implants can be positioned in conjunction with grafting procedures or after graft consolidation has occurred. Although it is difficult to determine a clear indication for immediate or delayed implant placement, most authors suggest immediate implant placement when the residual alveolar bone presents adequate quality and quantity [18].
Conclusion
Various surgical techniques have been developed to rehabilitate horizontally and vertically atrophied alveolar ridges. Autogenous block bone grafts can predictably restore function and esthetics of the atrophic anterior ridge. The modified shell technique for hard tissue grafting is a reasonable reconstruction method of the alveolar crest with vital bone. The overall morbidity of mandibular ramus block graft is minimal, and serves as a good alternative for ridge augmentation.
■Authors’ contributions
Sghaier Jihed has written the manuscript and managed the patient surgically with Abir Charfeddine. Afef Slim has participated in the design, the acquisition of clinical data as well as the revision of the manuscript. Abdelkader Smida; participated in the collection of bibliographic data. The other authors discussed the manuscript by revising it critically for important intellectual content and have given final approval for the version to be published. All authors approved the final draft of the manuscript.
■Ethics statement
“Written informed consent was obtained from the patient to publish this report by the journal’s patient consent policy”
Before starting our treatment plan, we received the informed consent of the patient, we informed him about the usefulness of the examinations performed, the diagnosis retained after these examinations, the detailed treatment plan while indicating the advantages and disadvantages of the treatment, and the possible side effects, as well as this work, will be used in a published scientific article. This consent was delivered before the date of surgery for proper reading and signing.
■Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
■Financial support
None
References
- Misch C. M. Autogenous Bone is Still the Gold Standard of Graft Materials in 2022. J Oral Implantol. 48, 169–170 (2022). [Google Scholar] [CrossRef]
- Sakkas, A., Wilde, F., Heufelder, M., et al. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. IntJ Implant Dent. 3, 1-17 (2017). [Google Scholar] [CrossRef]
- Khoury F, Khoury CH. Mandibular bone block grafts: instrumentation, harvesting technique and application. J Par Impl Orale; 25:15–34 (2006). [Google Scholar]
- Tan, W. L., Wong, T. L., Wong, M. C., et al. A systematic review of post‐extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 23, 1-21 (2012). [Google Scholar] [CrossRef]
- Aludden, H. C., Mordenfeld, A., Hallman, M., et al. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: a systematic review. Int J Oral Maxillofac Surg. 46, 1030-1038 (2017). [Google Scholar] [CrossRef]
- Sanz-Sánchez, I., Ortiz-Vigon, A., Sanz-Martín, I., et al. Effectiveness of lateral bone augmentation on the alveolar crest dimension: a systematic review and meta-analysis. J Dent Res 94(9_suppl), 128S-142S (2015). [Google Scholar] [CrossRef]
- Kuchler, U., & von Arx, T. Horizontal ridge augmentation in conjunction with or prior to implant placement in the anterior maxilla: a systematic review. Int J Oral Maxillofac Implants. 29 (2014). [Google Scholar] [CrossRef]
- Stricker, A., Schramm, A., Marukawa, E., et al. Distraction osteogenesis and tissue engineering--new options for enhancing the implant site. Int J Periodontics Restor. Dent. 23 (2003). [Google Scholar] [CrossRef]
- Fretwurst, T., Nack, C., Al-Ghrairi, et al. Long-term retrospective evaluation of the peri-implant bone level in onlay grafted patients with iliac bone from the anterior superior iliac crest. J Cranio-Maxillofac Surg. 43, 956-960 (2015). [Google Scholar] [CrossRef]
- Von Arx, T., & Buser, D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 17, 359-366 (2006). [Google Scholar] [CrossRef]
- Andersson, L. Patient self‐evaluation of intra‐oral bone grafting treatment to the maxillary frontal region. Dent Traumatol. 24, 164-169 (2008). [Google Scholar] [CrossRef]
- Zins, J. E., & Whitaker, L. A. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 72, 778-784 (1983). [Google Scholar] [CrossRef]
- Rabie, A. B. M., Dan, Z., & Samman, N. Ultrastructural identification of cells involved in the healing of intramembranous and endochondral bones. Int J Oral Maxillofac Surg. 25, 383-388 (1996). [Google Scholar] [CrossRef]
- Rocchietta, I., Fontana, F., & Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 35, 203-215 (2008). [Google Scholar] [CrossRef]
- Khoury, F., & Hanser, T. Mandibular bone block harvesting from the retromolar region: a 10-year prospective clinical study. Int J Oral Maxillofac Implants. 30, 688-697(2015). [Google Scholar] [CrossRef]
- Cordaro, L., Torsello, F., Morcavallo, S., et al. Effect of bovine bone and collagen membranes on healing of mandibular bone blocks: a prospective randomized controlled study. Clin Oral Implants Res. 22, 1145-1150 (2011). [Google Scholar] [CrossRef]
- Gibney, K. (2007). Bone augmentation in oral implantology. [Google Scholar] [CrossRef]
- Chiapasco, M., Zaniboni, M., & Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 17, 136-159(2006). [Google Scholar] [CrossRef]
- Ozaki, W., & Buchman, S. R. Volume maintenance of onlay bone grafts in the craniofacial skeleton: micro-architecture versus embryologic origin. Plast Reconstr Surg. 102, 291-299 (1998). [Google Scholar] [CrossRef]
- Raghoebar, G. M., Batenburg, R. H., Vissink, A., et al. Augmentation of localized defects of the anterior maxillary ridge with autogenous bone before insertion of implants. J Oral Maxillofac Surg. 54, 1180-1185 (1996). [Google Scholar] [CrossRef]
- Burchardt, H. The biology of bone graft repair. Clin Orthop Relat Res. 174, 28-34 (1983). [Google Scholar] [CrossRef]
- Chappuis, V., Cavusoglu, Y., Buser, D., & von Arx, T. Lateral ridge augmentation using autogenous block grafts and guided bone regeneration: A 10‐year prospective case series study. Clin Implant Dent Relat. Res. 19, 85-96 (2017). [Google Scholar] [CrossRef]
- Meijndert, C. M., Raghoebar, G. M., Meijndert, L., et al. Single implants in the aesthetic region preceded by local ridge augmentation; a 10‐year randomized controlled trial. Clin Oral Implants Res. 28, 388-395 (2017). [Google Scholar] [CrossRef]
- Sjöström, M., Sennerby, L., Nilson, H., & Lundgren, S. Reconstruction of the atrophic edentulous maxilla with free iliac crest grafts and implants: a 3‐year report of a prospective clinical study. Clin. Implant Dent Relat Res. 9, 46-59 (2007). [Google Scholar] [CrossRef]
- Miller, N., Penaud, J., Foliguet, B., et al. Resorption rates of 2 commercially available bioresorbable membranes: a histomorphometric study in a rabbit model. J Clin Periodontol. 23, 1051-1059 (1996). [Google Scholar] [CrossRef]
- Cordaro, L., Amadè, D. S., & Cordaro, M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 13, 103-111 (2002). [Google Scholar] [CrossRef]
- Acocella A, Bertolai R, Colafranceschi M, et al. Clinical, histological and histomorphometric evaluation of the healing of mandibular ramus bone block grafts for alveolar ridge augmentation before implant placement J Cranio-Maxillofac Surg.;38:222-230 (2010). [Google Scholar] [CrossRef]
- Marx, R. E. Bone and bone graft healing. Oral Maxillofac Surg Clin N Am. 19(4), 455-466 (2007). [Google Scholar] [CrossRef]
- Kennedy, J. E. Effect of inflammation on collateral circulation of the gingiva. Journal Periodontal Res. 9, 147-152 (1974). [Google Scholar] [CrossRef]
- Evans, C. D., & Chen, S. T. Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 19, 73-80 (2008). [Google Scholar] [CrossRef]
- Schwartz-Arad, D., Levin, L., & Sigal, L Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation. Implant Dent. 14, 131-138. (2005). [Google Scholar] [CrossRef]
- Altiparmak, N., Soydan, S. S., & Uçkan, S. The effect of conventional surgery and piezoelectric surgery bone harvesting techniques on the donor site morbidity of the mandibular ramus and symphysis. Nt J Oral Maxillofac Surg. 44, 1131-1137 (2015). [Google Scholar] [CrossRef]
- Clavero, J., & Lundgren, S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin. Implant Dent Relat Res. 5, 154-160 (2003). [Google Scholar] [CrossRef]
- Misch, C. M. Autogenous bone: is it still the gold standard?. Implant Dent. 19, 361 (2010). [Google Scholar] [CrossRef]